Introduction
Obesity is considered one of the most serious health problems in the world. The abundance and the use of energy-dense and high calories foods, smoke, stress and a sedentary lifestyle, lead to obesity, with 2 billion of people in the world considered obese and/or overweight (WHO) [1]. Obesity is also considered a major risk factor for metabolic syndrome (MetS), characterised by hyperinsulinemia, hyperglycaemia, hyperlipidaemia and hepatic disorders, such as non-alcoholic fatty liver disease (NAFLD) [2].
Oxidative stress (OS) plays an important role in the development of obesity associated diseases and obese individuals are characterised by higher levels of oxidative stress compared to lean people [3] and lower anti-oxidant defences [4]. An excess of reactive oxygen species (ROS) combined with a low anti-oxidant capacity in the cells has been suggested to promote the development of obesity-induced metabolic diseases [5]. In metabolic diseases, OS is caused by different factors including mitochondrial dysfunction, activation of ROS and nitrogen species (RNS) producing enzyme, accumulation of glucose, lipids and protein oxidation products [6]. Moreover, metabolic diseases are associated with chronic low-grade inflammation (CLGI) [7], producing abnormal pro-inflammatory cytokines, and activating inflammatory signalling pathways [8]. Inflammation is promoted by the presence, in the enlarged adipose tissue, of macrophages and immune cells, such as lymphocytes T [9]. Adipocytes and T cells have similar roles in complementary activation of inflammatory pathways and production of inflammatory cytokines: in fact, adipocyte precursors can be transformed into macrophage-like cell thanks to the phagocytic capacity under specific stimuli [10]. Some of the most important molecules involved in obesity-derived inflammation processes are tumour necrosis factor α (TNF-α) [11], interleukin 1β and 6 (IL1β, IL-6) [12,13], leptin, adiponectin and Janus kinase 3 (JAK3) [14,15].
Due to the high number of obese people, different strategies and new protocols to fight the onset of this obesity epidemic and the increased incidence of associated co-morbidities are required. A balanced diet and proper physical activity are the basis of these strategies, however improving redox status in obese people is of paramount importance. Healthy foods, rich in antioxidant and anti-inflammatory molecules have a role; yet, it is necessary to consider supplements or nutraceuticals that can increase biological activity against ROS and inflammatory state, and improve redox status.
Astaxanthin (ASX), called also 3,3′-dihydroxy-β, β′-carotene-4,4′-dione, is a secondary carotenoid belonging to xanthophyll family [16,17]. ASX is ubiquitous in nature, in fact it can be produced by plants, bacteria and yeast [18], but one of the highest producer is Haematococcus pluvialis, a unicellular freshwater green microalga [19]. ASX structure is characterised by keto and hydroxyl group at the end of the molecule, which make ASX one of the most powerful antioxidant compounds.
ASX, as antioxidant, has ten times higher activity than other carotenoids (e.g. β-carotene, lutein and zeaxanthin) and hundred times than α-tocopherol [16,17]. Furthermore, ASX differs from carotenoids in its metabolism: ASX is absorbed by the intestinal mucosa through passive diffusion and is carried to the liver via the lymphatic and blood system, enclosed in chylomicrons [20]. The difference between carotenoids and ASX mainly lies in the type of lipoprotein that carries them once metabolized by the liver. Carotenoids are redistributed in plasma through low-density lipoproteins (LDL), whereas ASX is equally divided between LDL lipoproteins and high-density lipoproteins (HDL) [21]. Very few studies have been conducted on the pharmacokinetics of ASX. Choi et al. reported that ASX is unstable to gastric juices and that oral absorption is dose-independent and follows a flip-flop model, unlike intravenous, which is dose-dependent [22]. Given the high instability of ASX, Ødeberg et al. suggested the use of lipid formulations to improve its absorption for potential use in clinical trials [23].
Few studies have been conducted on ASX and its effect on human metabolic disease. Mashhaid et al. reported, in their studies, that ASX plays an important role in reducing level of triglycerides, cholesterol and blood pressure in type 2 diabetes (T2D) patients [24]; Choi et al. showed that ASX improved oxidative stress biomarker activity in obese adults [25] and Chen et al. reported that ASX had anti-coagulant effects in T2D patients reducing level of plasminogen activator inhibitor (PAI)-1 and anticoagulant factor VII (FVII) [26]. Furthermore, ASX has been shown to have some effects against obesity associated diseases in animal models and descriptive results and potential mechanisms of action have been reviewed by Bonet et al. [27]; however no systematic analysis of all the available data has been carried out to date. A systematic review and meta-analysis of animal studies would, therefore, provide useful information for the design of subsequent human clinical studies for the use of ASX as a supplement or nutraceutical. This systematic review and meta-analysis aimed to comprehensively examine the effects of ASX in animal models (mice or rats) of diet induced obesity-associated diseases, focusing specifically on MetS, NAFLD and T2D.
Section snippets
Methods
A systematic search for English-language manuscripts, published between January 2000 and April 2020, was made using five databases: Cinahl, Cochraine, MEDLINE, Scopus and Web of Science. The key words “Astaxanthin, obesity, non-alcoholic fatty liver disease, nonalcoholic fatty liver disease, diabetes, diabetes mellitus, type 2, NAFLD and metabol*” were used in each database and the exact strings used for each data base are reported in Table 1. The results are reported in accordance with PRISMA
Search results
A total of 506 articles (Cinahl 107; Cochraine 27; MEDLINE 153; Scopus 116; WOS 103) were found and, after removing duplicates, 312 articles were selected for the next step. By screening title and abstract of the selected articles, reviews, cell studies and human studies were removed and 39 articles were selected for full text screening. Based on inclusion and exclusion criteria as described above, 17 articles were selected for inclusion in the review (Fig. 1), which included 21 animal studies.
Discussion
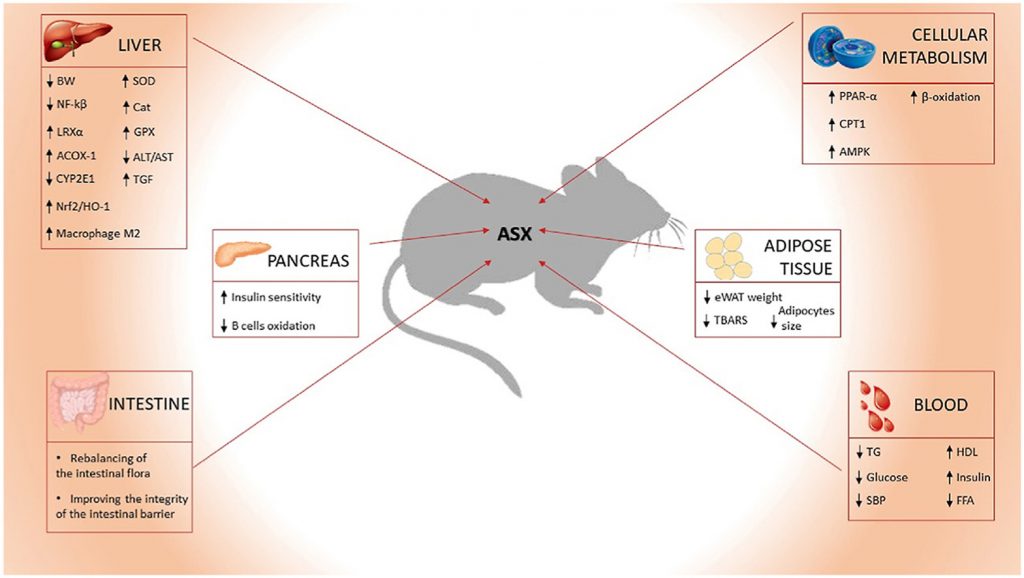
This review aimed to systematically review the effect that ASX had on pathological conditions, such as MetS, T2D and NAFLD, caused by an unbalanced diet (e.g. HFD, HFFD, HF/HS diet) in different animal models. It also analysed how different ASX concentrations influenced different biomarkers of disease in control animals or with disease phenotype.
In relation to biomarkers of metabolic syndrome, ASX, at different concentrations and administered for different length of time, induced a significant
Authors’ contribution
RPR and GB conceptualized the study, developed the protocol, selected articles for full-text review. RPR extracted data from the included studies, and RPR and GB performed all statistical analyses. RPR, ARL, EV, MCP, GM and GB wrote and reviewed the manuscript.
Declaration of competing interest
None.
Acknowledgments
RPR was supported by a fellowship under the Erasmus Traineeship Program from the University of Basilicata during her stay in Aberdeen.